Therapeutic protein solutions are subject to interfaces (container materials and air) during storage and in preparation and administration protocols. The interface with air can be dynamic during agitation when the meniscus of the solution sweeps the walls of the container. In this case, proteins adsorbed on the surface of the container, are alternately in air and in solution. Proteins can thus be subject to repeated partial déhydration.
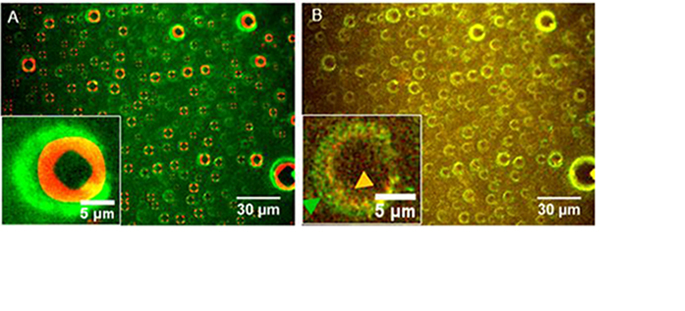
Thanks to the design of a dynamic and controlled triple interface in a microfluidic channel, we observed in real time by fluorescence and interference microscopy that insulin, adsorbed on a hydrophobic surface, aggregates into amyloid fibers under the effect of repeated dehydration. Insulin aggregates form at the border of droplets, at the triple interface, and grow eccentrically around the droplets. This work has direct implications on the manipulation protocols of formulations containing therapeutic proteins in order to preserve their stability. This subject is at the heart of the LabCom LMGP EVEON.
This work is published in Frachon T, Bruckert F, Masne Q, Monnin E and Weidenhaupt M (2016) Insulin aggregation at a dynamic solid-liquid-air interface. Langmuir 32 (49), 13009-13019.