Publication d'Elisa Migliorini 2017
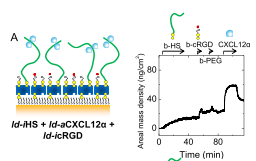
Le papier "Binding of the chemokine CXCL12? to its natural extracellular matrix ligand heparan sulfate enables myoblast adhesion and facilitates cell motility" a été publié dans Biomaterials
Ici vous trouverez le papier d'Elisa Migliorini