-
- au
Ici vous trouverez le papier de David Muñoz-Rojas
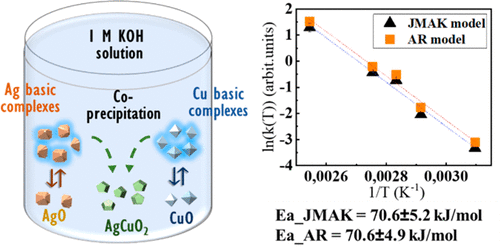
"AgCuO2 is an interesting semiconductor oxide with appealing optical and electronic properties. While the oxide has been synthesized in bulk by different approaches, no study of the formation mechanism has been carried out to date. We present an in situ time-resolved X-ray diffraction study of the hydrothermal synthesis of AgCuO2 from AgO and CuO. The effects of reaction pH and temperature on the reaction pathways and products have been studied. While the pH is a key parameter for the successful synthesis of AgCuO2, the temperature affects mainly the reaction kinetics. A reaction pathway is proposed that involves a series of dissolution–precipitation reactions, mediated by Cu and Ag hydroxy complexes. Finally, we have compared different approaches to obtain the reaction activation energy, which was calculated to be 70.6 ± 5.1 kJ/mol. Nevertheless, our results show that new models need to be developed for the type of reaction presented here."


