-
- au
Ici vous trouverez le papier de Clement Lausecker
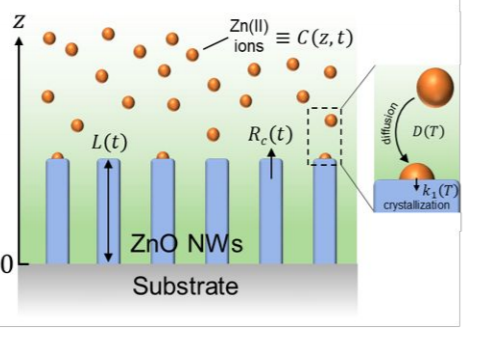
".The chemical bath deposition of nanowires is of high interest for a wide variety of optoelectronic, piezoelectric, and sensing devices, but a theoretical description of the elongation process is still missing despite its critical importance. By solving Fick’s diffusion equations in combination with thermodynamic computations, we determine the expression of the axial growth rate of nanowires and its temporal dependence under dynamic conditions, namely in a sealed reactor where the depletion of chemical reactants occurs. The theoretical model is found to be in very good agreement with a large set of experimental data specifically collected in the case of the chemical bath deposition of ZnO nanowires. In particular, an activation energy of 198 ± 24 kJ/mol is deduced for the elongation process of ZnO nanowires, involving the energy barriers for both the dehydration process of Zn(II) species (i.e. [Zn(H2O)6]2+ ions) and their subsequent direct incorporation onto the c-plane top faces. This shows its high potential for deeply investigating the physicochemical processes at work in the chemical bath. By using the theoretical model as a predictive approach, a complete growth diagram reporting the evolution of the length of ZnO nanowires vs effective growth time and temperature is also gained over a broad range of conditions, revealing its additional high potential for applied research and industrial purposes. The present general approach is further compatible with a broad range of chemicals in solution and of semiconducting materials grown by chemical bath deposition"


