Here you will find the article by
José Villafuerte:
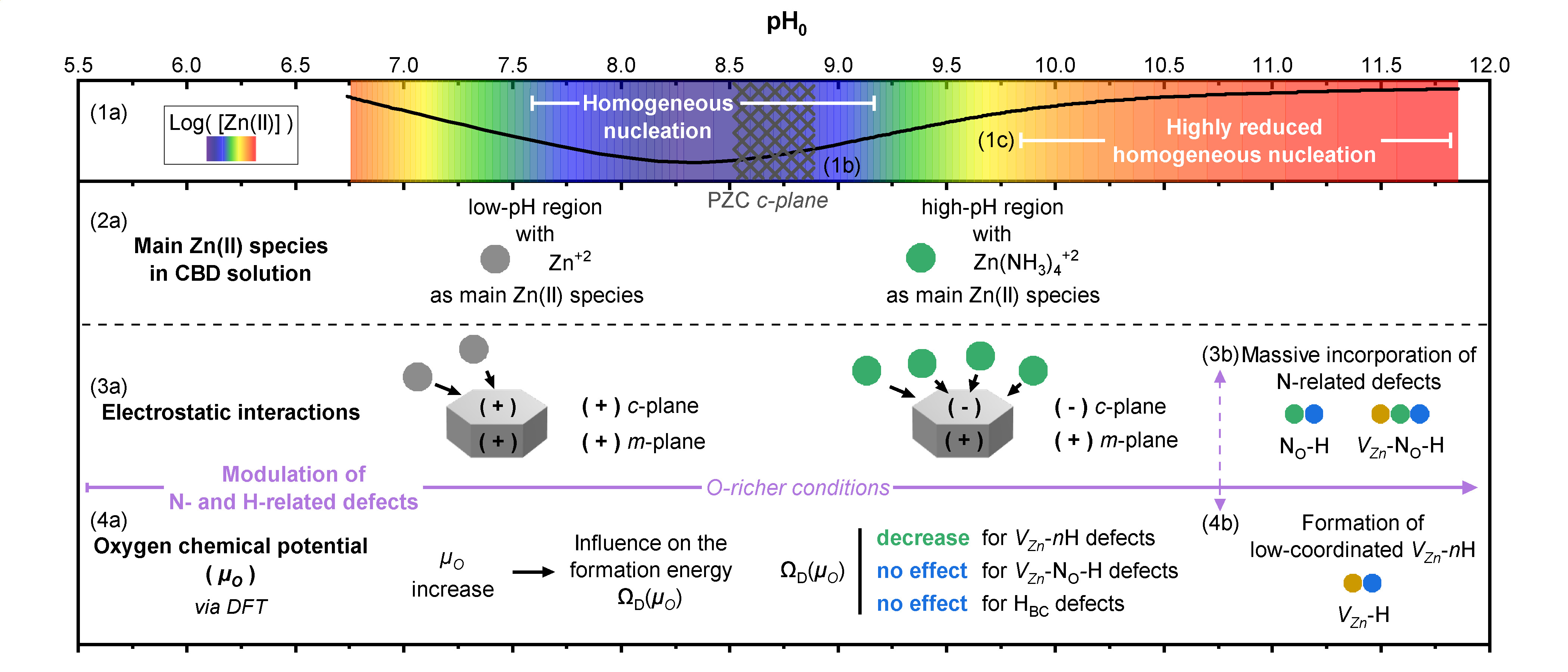
"ZnO nanowires (NWs) grown by chemical bath deposition (CBD) have received a great interest for nanoscale engineering devices, but their formation in aqueous solution containing many impurities needs to be carefully addressed. In particular, the pH of the CBD solution and its effect on the formation mechanisms of ZnO NWs and of nitrogen- and hydrogen-related defects in their center are still unexplored. By adjusting its value in a low- and high-pH region, we show the latent evolution of the morphological and optical properties of ZnO NWs, as well as the modulated incorporation of nitrogen- and hydrogen-related defects in their center using Raman and cathodoluminescence spectroscopy. The increase in the pH is related to the increase in the oxygen chemical potential (µ
O), for which the formation energy of hydrogen in bond-centered sites (H
BC) and V
Zn-N
O-H defect complexes is found to be unchanged, whereas the formation energy of zinc vacancy (V
Zn) and zinc vacancy-hydrogen (V
Zn-nH) complexes steadily decreases as shown from density-functional theory calculations. Revealing that these V
Zn-related defects are energetically favorable to form as µ
O is increased, ZnO NWs grown in the high-pH region are found to exhibit a higher density of V
Zn-nH defect complexes than ZnO NWs grown in the low-pH region. Annealing at 450 °C under oxygen atmosphere helps tuning the optical properties of ZnO NWs by reducing the density of H
BC and V
Zn-related defects, while activating the formation of V
Zn-N
O-H defect complexes. These findings show the influence of pH on the nature of Zn(II) species, the electrostatic interactions between these species and ZnO NW surfaces, and the formation energy of the involved defects. They emphasize the crucial role of the pH of the CBD solution and open new possibilities for simultaneously engineering the morphology of ZnO NWs and the formation of nitrogen- and hydrogen-related defects."