-
- to
Here you will find the paper by Françoise Hippert
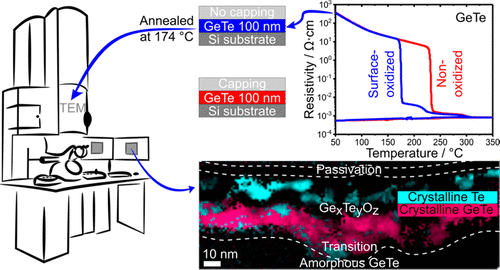
" The outstanding properties of chalcogenide phase-change materials (PCMs) led to their successful use in innovative resistive memory devices where the material is switched between its amorphous and crystalline phases. However, PCMs are easily oxidized at interfaces. Oxidation is detrimental to device performances. In particular, it reduces the data retention time since oxidized PCMs crystallize at a lower temperature than nonoxidized ones. The aim of this study is to investigate how oxidation affects the crystallization process of germanium telluride (GeTe), a prototypical PCM. By using advanced scanning transmission electron microscopy (STEM) techniques, including spatially resolved correlations between composition maps measured by energy-dispersive X-ray (EDX) spectroscopy and structural information deduced from electron diffraction patterns and high-resolution X-ray photoelectron spectroscopy, we obtained a thorough description of the local chemistry and structure of an oxidized GeTe thin film, partly crystallized by heating an initially amorphous film at ∼180 °C. Under an oxide layer consisting of amorphous GeOx and TeOx, the upper part (∼30–40 nm thick) of the film consists of segregated amorphous GeOx, crystalline GeTe, and, strikingly, pure Te crystallites. The bottom part of the film, in which no oxygen has penetrated, stayed amorphous. This study reveals why oxidation promotes crystallization of GeTe through segregation of Te regions and heterogeneous nucleation. These results explain why oxidation at the surface or interfaces reduces the crystallization temperature of GeTe (by 50 °C with respect to a nonoxidized material) and shed light on the major impact of interface chemistry on the crystallization mechanism of PCMs used in resistive memory devices. "


