Publication de Masoud Akbari
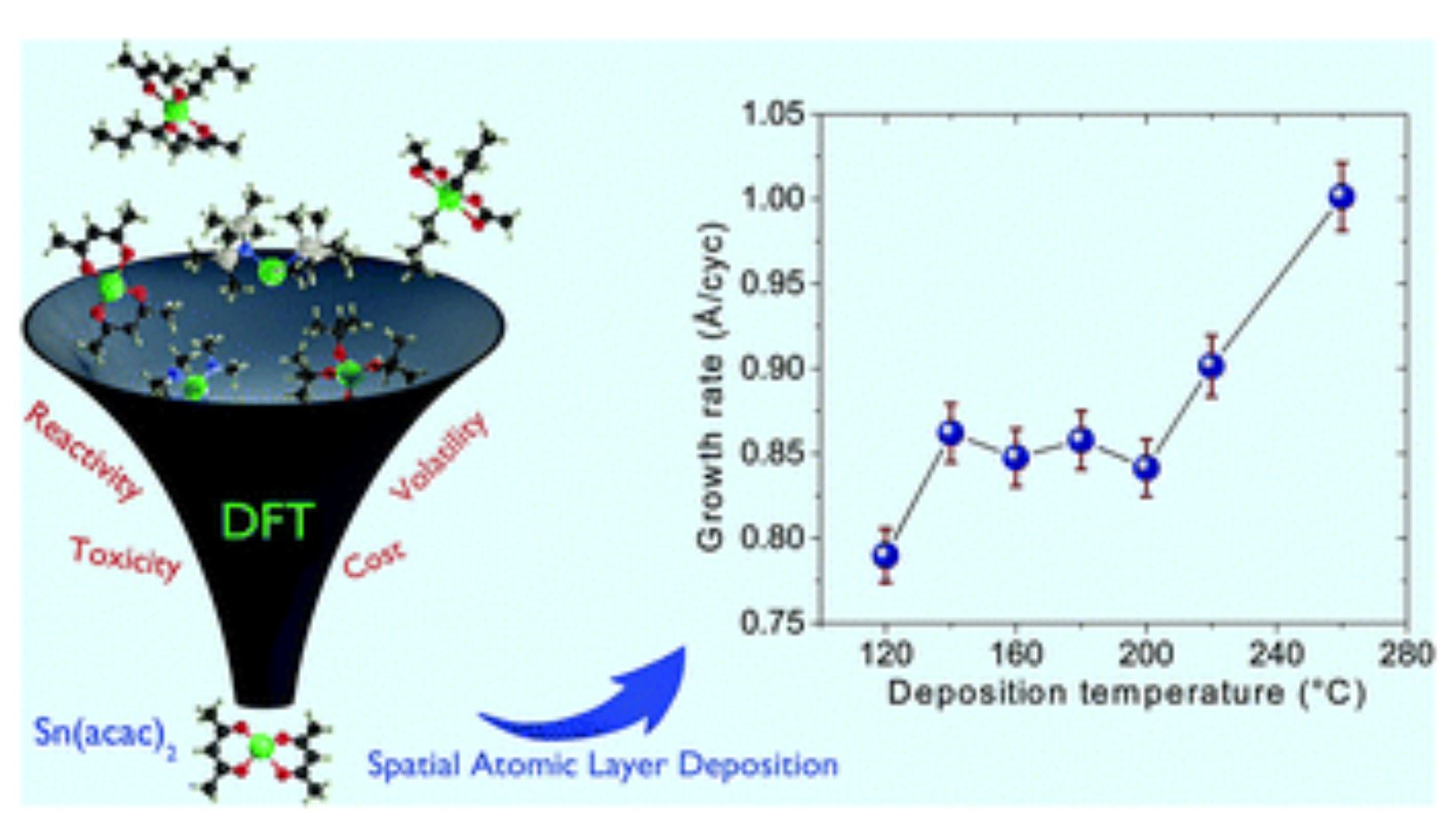
L'article intitulé "Atmospheric atomic layer deposition of SnO2 thin films with tin(ii) acetylacetonate and water" a été publié dans Dalton Transactions faisant partie de: Spotlight Collection: Atomic and Molecular Layer Deposition